
Business Development Spotlight with Christopher Bjerkan Wade
Discover more about our team. In this Spotlight, meet our Senior Business Development Director, Christopher Bjerkan Wade.
Discover a CDMO with a science-led approach and unparalleled project delivery.

As a leading Contract, Development and Manufacturing Organisation (CDMO) our defining traits lie in our adaptability and nimbleness.
We align to your drug development needs from pre-clinical to late phase manufacture, with a science-led approach that delivers across oral solids, nasal, pulmonary and parenteral dosage forms.
Everything you need from pre-clinical to market, all in one place.

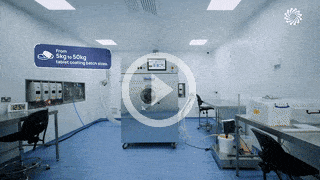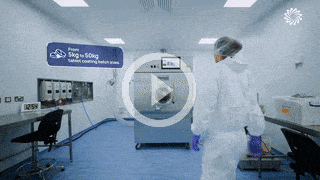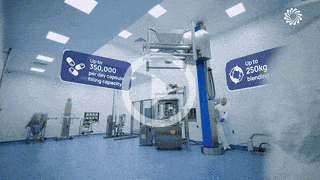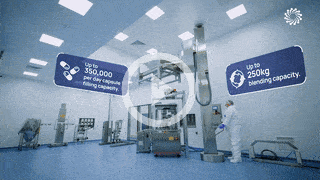
Our team support you at each stage of your drug program lifecycle from pre-clinical to market.
Our CDMO services include formulation development, phase 1, phase 2 and phase 3 clinical supply, process scale-up and optimisation, quality control, analytical development and validation, and registration activities.
We keep the knowledge of your molecule within the same project team, from start to finish.
With oversight from an Executive Leadership Team member on every project, our approach ensures continuity and access to subject matter expertise from across our business.
Science-led, quick decision-making and collaboration keep your project moving forward.



Discover more about our team. In this Spotlight, meet our Senior Business Development Director, Christopher Bjerkan Wade.

Upperton announces its recognition as winner of the Small Molecule Dosage Form category at the inaugural European CDMO Leadership Awards.
Trent Gateway, Technology Drive, Beeston, Nottingham, NG9 1LA
If you’re looking to work with a CDMO that can support your product from pre-clinical development to market, then we’re here to help.
Download our brochure to learn more about our capabilities.
Speak with our team of experts and set up a discovery call to discuss your project in more detail.
What Biotechs Should Be Looking For Next